Abstract
Introduction: Burkitt lymphoma (BL) accounts for approximately 50% of all pediatric non-Hodgkin lymphomas compared to 1-2% in adults. Adult BL (aBL) remains a poorly understood entity and its relationship to pediatric BL (pBL) and to DLBCL has not been fully elucidated. The variable treatment outcomes between these entities necessitate a more thorough understanding of the genetic and molecular features underlying their biology to enable better prognostication and more effective treatments. We sought to comprehensively determine genetic features shared with DLBCL and those that are unique to BL, to further delineate genetic subgroupings within each entity.
Methods: Samples for this study were collected through the Burkitt Lymphoma Genome Sequencing Project (BLGSP). We sequenced the tumor genomes of 139 pBL and 92 aBL, consisting of both EBV-positive (EBV+) and EBV-negative (EBV-) BLs, and compared these to the genomes of 252 DLBCL patients. All cases were analyzed for simple somatic mutations (SSM), recurrent copy number variations (CNV), structural variations (SV), aberrant somatic hypermutation (aSHM), and SSM hotspots. Mutations were used as features for the identification of genetic subgroups using non-negative matrix factorization (NMF) clustering.
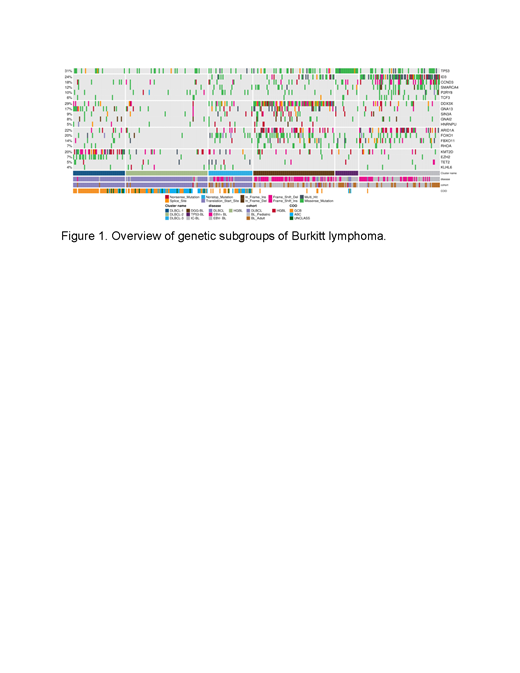
Results: Clustering of BL and DLBCL revealed six distinct genetic subgroups (Figure 1) with three primarily representing DLBCLs (DLBCL-1, DLBCL-2, and DLBCL-3) and three predominantly comprising BLs (M53-BL, IC-BL, and DGG-BL). The DLBCL-predominant subgroups partially overlapped with those previously described and resembled features of EZB and ST2-like subgroups. The frequency of aBLs within these subgroups was higher than that of pBL patients (p=0.0005). The new cluster M53-BL consists of both pBL (9/27) and aBL (13/27) samples and is characterized by the highest prevalence of mutations in TP53 accompanied by the paucity of other driver mutations but without the aneuploidy associated with the A53 subgroup described in DLBCL. Enrichment of EBV- samples in this cluster further corroborate our previous findings of TP53 mutations being associated with EBV- BL. IC-BL is characterized by mutations in ID3, CCND3, and SMARCA4. In contrast, DGG-BL, where 65% of the cluster consisted of EBV+ BL samples, had mutations in DDX3X, GNA13, and GNAI2. Using a linear model, we compared the rates of aSHM in BL genomes from all clusters and identified the DLBCL-3 cluster to harbor the highest aSHM rates at common sites while the M53-BL cluster harbored the lowest rates. To further establish the biological basis of unique clusters within BL, we conducted differential gene expression analyses between the two major BL genetic subgroups, DGG-BL and IC-BL. We identified a total of 86 differentially expressed genes between the two clusters (p.adj < 0.01 and |log2foldChange| > 1). Among the genes with the strongest differential expression were IRF4, SERPINA9, and TNFRSF13B. Each of these are notable as their expression is a component of the DLBCL cell-of-origin and double-hit signature classifiers. Further, we found IRF4 expression to be one of the strongest predictors of cluster membership, with high IRF4 expression associated with IC-BL membership. Using TP53 and ID3 mutations as a proxy for M53-BL and IC-BL clusters in aBL, we found mutations in TP53 to be associated with significantly inferior progression free survival (PFS) at 2 year follow up, while mutations in ID3 were associated with overall better PFS at 2 year follow up.
Conclusion: This work identifies novel genetic subgroups within BL with characteristic genetic and gene expression differences and some bearing relationship to DLBCL subgroups. The three subgroups with predominantly BL samples (DGG-BL, IC-BL, and M53-BL) each comprised a mixture of aBL and pBL samples, confirming similar molecular features in these entities. The IC-BL cluster is associated with mutations in ID3 and CCND3, high IRF4 expression, and ID3 mutated cases exhibited significantly better outcomes. M53-BL is associated with TP53 mutations and inferior PFS in aBL, representing a subset of patients to be considered for novel treatment approaches. These findings highlight shared pathogenesis between aBL and pBL and establish genetic subtypes within BL that delineate cases with distinct molecular and clinical features. This provides a new framework for new diagnostic and therapeutic strategies.
Abramson: Seagen Inc.: Research Funding; Allogene Therapeutics: Consultancy; Astra-Zeneca: Consultancy; Incyte Corporation: Consultancy; BeiGene: Consultancy; Kymera: Consultancy; Kite Pharma: Consultancy; Novartis: Consultancy; Bluebird Bio: Consultancy; C4 Therapeutics: Consultancy; Morphosys: Consultancy; Genmab: Consultancy; EMD Serono: Consultancy; Bristol-Myers Squibb Company: Consultancy, Research Funding; AbbVie: Consultancy; Karyopharm: Consultancy; Genentech: Consultancy. Bartlett: Pharmacyclics: Research Funding; Millennium: Research Funding; Merck: Research Funding; Kite, a Gilead Company: Research Funding; Janssen: Research Funding; Genentech: Research Funding; Forty Seven: Research Funding; Celgene: Research Funding; Bristol Myers Squibb: Research Funding; Autolus: Research Funding; Seagen: Consultancy, Research Funding; Roche/Genentech: Consultancy; ADC Therapeutics: Consultancy, Research Funding. Casper: EUSA Pharma: Consultancy. Gerrie: Roche: Research Funding; AbbVie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Astrazeneca: Honoraria, Research Funding; Sandoz: Honoraria. Grande: Sage Bionetworks: Current Employment. Mullighan: Illumina: Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; AbbVie: Research Funding; Amgen: Current equity holder in publicly-traded company. Noy: Epizyme: Consultancy; Rafael Parhma: Research Funding; Morphosys: Consultancy; Targeted Oncology: Consultancy; Medscape: Consultancy; Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy, Honoraria. Scott: NanoString Technologies: Patents & Royalties: Patent describing measuring the proliferation signature in MCL using gene expression profiling.; AstraZeneca: Consultancy; Abbvie: Consultancy; Celgene: Consultancy; Incyte: Consultancy; Janssen: Consultancy, Research Funding; Rich/Genentech: Research Funding; BC Cancer: Patents & Royalties: Patent describing assigning DLBCL COO by gene expression profiling--licensed to NanoString Technologies. Patent describing measuring the proliferation signature in MCL using gene expression profiling. . Morin: Foundation for Burkitt Lymphoma Research: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Epizyme: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal